PREVNAR 20 4 Dosage and Administration
Pneumococcal 20-valent Conjugate Vaccine [Diphtheria CRM197 Protein]
4.1 Dosing Considerations
- Individuals at higher risk of pneumococcal infection, including patients with sickle cell disease (SCD) or human immunodeficiency virus (HIV) infection, and those previously vaccinated with one or more doses of the 23-valent pneumococcal polysaccharide vaccine (PPSV23), are recommended to receive at least one dose of PREVNAR 20 (see 7 Warnings and Precautions, Immune and 14 Clinical Trials, PREVNAR 13 Immune Responses in Special Populations).
- In individuals with a hematopoietic stem cell transplant (HSCT), the recommended immunization series with PREVNAR 20 consists of four doses of 0.5 mL. The primary series consists of three doses, with the first dose given 3 to 6 months after HSCT and with an interval of at least 1 month between doses. A booster dose is recommended 6 months after the third dose (see 7 Warnings and Precautions, Immune and 14 Clinical Trials, PREVNAR 13 Immune Responses in Special Populations).
- If the sequential use of PPSV23 is considered appropriate, PREVNAR 20 should be given first.
4.2 Recommended Dose and Dosage Adjustment
4.2.1 Pediatrics (6 Weeks Through 17 Years of Age)
It is recommended that infants who receive a first dose of PREVNAR 20 complete the vaccination series with PREVNAR 20.
Routine Vaccination Schedule for Infants and Toddlers 6 Weeks Through 15 Months of Age
4-Dose Series (3-Dose Primary Series Followed by a Toddler Dose)
The vaccination series consists of 4 doses of PREVNAR 20, each of 0.5 mL. The primary series consists of 3 doses, with the first dose usually given at 2 months of age (and as early as 6 weeks of age), with an interval of 4 to 8 weeks between doses. The fourth dose should be given between 11 and 15 months of age and at least 2 months after the third dose.
See 14.2.2 Clinical Trials in Infants, Children and Adolescents 6 Weeks Through 17 Years of Age for the 3-Dose Series (2-Dose Primary Series Followed by a Toddler Dose)
Pre-term Infants (<37 Weeks Gestation at Birth)
The recommended vaccination series consists of 4 doses of PREVNAR 20, each of 0.5 mL. The primary series consists of 3 doses, with the first dose usually given at 2 months of age (and as early as 6 weeks of age), with an interval of 4 to 8 weeks between doses. The fourth dose should be given between 11 and 15 months of age and at least 2 months after the third dose.
Catch-up Vaccination Schedule for Unvaccinated Children and Adolescents 7 Months Through 17 Years of Age
Children 7 months through 17 years of age who have never received a pneumococcal conjugate vaccine may receive PREVNAR 20 according to the following schedules:
Infants 7 Through 11 Months of Age
Three doses of 0.5 mL, with the first 2 doses given at least 4 weeks apart. The third dose is given after the 1-year birthday, separated from the second dose by at least 2 months.
Children 12 Through 23 Months of Age
Two doses of 0.5 mL, with an interval of 2 months between doses.
Children and Adolescents 2 Through 17 Years of Age
One single 0.5 mL dose.
Catch-up Vaccination Schedule for Children Previously or Incompletely Vaccinated with PREVNAR 13
Children 15 months through 17 years of age who are considered completely immunized or with an incomplete vaccine series of PREVNAR 13 may receive 1 single 0.5 mL dose of PREVNAR 20. The catch-up (supplemental) dose of PREVNAR 20 should be administered with an interval of at least 8 weeks after the final dose of PREVNAR 13.
4.2.2 Adults (18 Years of Age and Older)
PREVNAR 20 is administered intramuscularly as a single 0.5 mL dose.
4.4 Administration
Do not mix PREVNAR 20 with any other vaccines or products in the same syringe.
Preparation for administration
Step 1. Vaccine resuspension Hold the pre-filled syringe horizontally between the thumb and the forefinger and shake vigorously until the contents of the syringe are a homogeneous white suspension. Do not use the vaccine if it cannot be re‑suspended. | 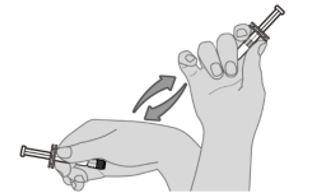 |
Step 2. Visual inspection Visually inspect the vaccine for large particulate matter and discoloration prior to administration. Do not use if large particulate matter or discoloration is found. If the vaccine is not a homogeneous white suspension, repeat steps 1 and 2. | 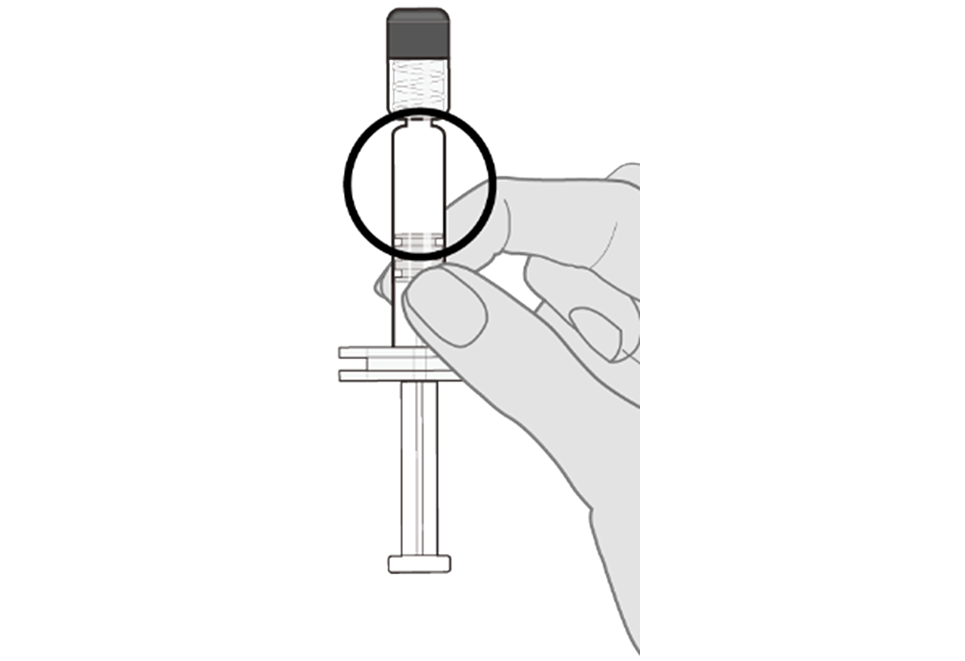 |
Step 3. Remove syringe cap Remove the syringe cap from the Luer lock adapter by slowly turning the cap counter‑clockwise while holding the Luer lock adapter.
Note: Care should be taken to ensure that the extended plunger rod is not depressed while removing the syringe cap. | 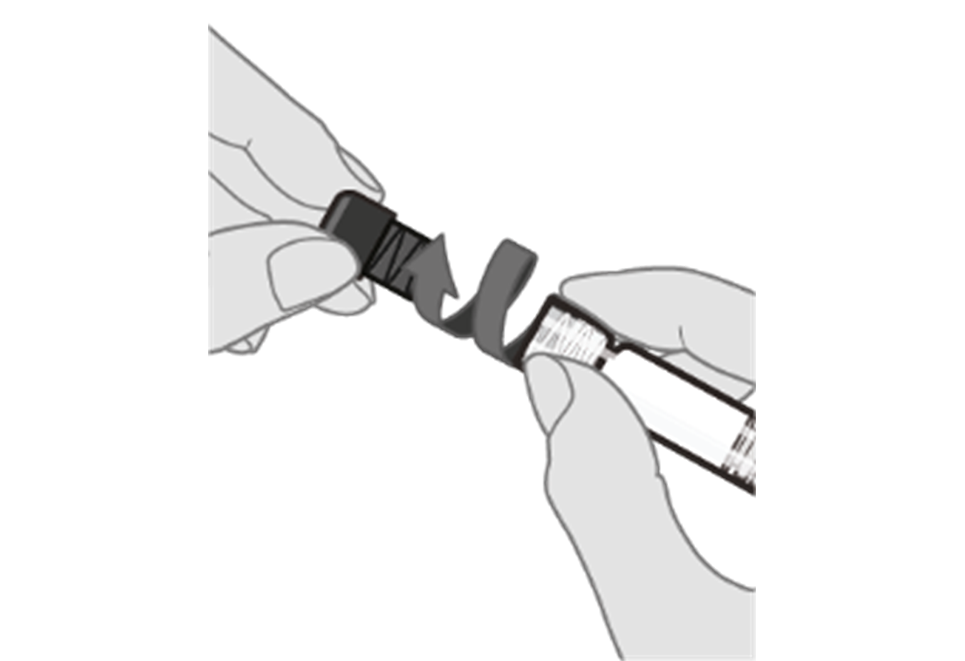 |
Step 4. Attach a sterile needle Attach a needle appropriate for intramuscular administration to the pre-filled syringe by holding the Luer lock adapter and turning the needle clockwise. | |
Administration
For intramuscular use only.
Each 0.5 mL dose is to be injected intramuscularly, with care to avoid injection into or near nerves and blood vessels. The preferred sites for injection are the anterolateral aspect of the thigh in infants and the deltoid muscle of the upper arm in children and adults. The vaccine should not be injected in the gluteal area.
Do not administer PREVNAR 20 intravascularly.
)Find PREVNAR 20 medical information:
Find PREVNAR 20 medical information:
PREVNAR 20 Quick Finder
Health Professional Information
4 Dosage and Administration
4.1 Dosing Considerations
- Individuals at higher risk of pneumococcal infection, including patients with sickle cell disease (SCD) or human immunodeficiency virus (HIV) infection, and those previously vaccinated with one or more doses of the 23-valent pneumococcal polysaccharide vaccine (PPSV23), are recommended to receive at least one dose of PREVNAR 20 (see 7 Warnings and Precautions, Immune and 14 Clinical Trials, PREVNAR 13 Immune Responses in Special Populations).
- In individuals with a hematopoietic stem cell transplant (HSCT), the recommended immunization series with PREVNAR 20 consists of four doses of 0.5 mL. The primary series consists of three doses, with the first dose given 3 to 6 months after HSCT and with an interval of at least 1 month between doses. A booster dose is recommended 6 months after the third dose (see 7 Warnings and Precautions, Immune and 14 Clinical Trials, PREVNAR 13 Immune Responses in Special Populations).
- If the sequential use of PPSV23 is considered appropriate, PREVNAR 20 should be given first.
4.2 Recommended Dose and Dosage Adjustment
4.2.1 Pediatrics (6 Weeks Through 17 Years of Age)
It is recommended that infants who receive a first dose of PREVNAR 20 complete the vaccination series with PREVNAR 20.
Routine Vaccination Schedule for Infants and Toddlers 6 Weeks Through 15 Months of Age
4-Dose Series (3-Dose Primary Series Followed by a Toddler Dose)
The vaccination series consists of 4 doses of PREVNAR 20, each of 0.5 mL. The primary series consists of 3 doses, with the first dose usually given at 2 months of age (and as early as 6 weeks of age), with an interval of 4 to 8 weeks between doses. The fourth dose should be given between 11 and 15 months of age and at least 2 months after the third dose.
See 14.2.2 Clinical Trials in Infants, Children and Adolescents 6 Weeks Through 17 Years of Age for the 3-Dose Series (2-Dose Primary Series Followed by a Toddler Dose)
Pre-term Infants (<37 Weeks Gestation at Birth)
The recommended vaccination series consists of 4 doses of PREVNAR 20, each of 0.5 mL. The primary series consists of 3 doses, with the first dose usually given at 2 months of age (and as early as 6 weeks of age), with an interval of 4 to 8 weeks between doses. The fourth dose should be given between 11 and 15 months of age and at least 2 months after the third dose.
Catch-up Vaccination Schedule for Unvaccinated Children and Adolescents 7 Months Through 17 Years of Age
Children 7 months through 17 years of age who have never received a pneumococcal conjugate vaccine may receive PREVNAR 20 according to the following schedules:
Infants 7 Through 11 Months of Age
Three doses of 0.5 mL, with the first 2 doses given at least 4 weeks apart. The third dose is given after the 1-year birthday, separated from the second dose by at least 2 months.
Children 12 Through 23 Months of Age
Two doses of 0.5 mL, with an interval of 2 months between doses.
Children and Adolescents 2 Through 17 Years of Age
One single 0.5 mL dose.
Catch-up Vaccination Schedule for Children Previously or Incompletely Vaccinated with PREVNAR 13
Children 15 months through 17 years of age who are considered completely immunized or with an incomplete vaccine series of PREVNAR 13 may receive 1 single 0.5 mL dose of PREVNAR 20. The catch-up (supplemental) dose of PREVNAR 20 should be administered with an interval of at least 8 weeks after the final dose of PREVNAR 13.
4.2.2 Adults (18 Years of Age and Older)
PREVNAR 20 is administered intramuscularly as a single 0.5 mL dose.
4.4 Administration
Do not mix PREVNAR 20 with any other vaccines or products in the same syringe.
Preparation for administration
Step 1. Vaccine resuspension Hold the pre-filled syringe horizontally between the thumb and the forefinger and shake vigorously until the contents of the syringe are a homogeneous white suspension. Do not use the vaccine if it cannot be re‑suspended. |  |
Step 2. Visual inspection Visually inspect the vaccine for large particulate matter and discoloration prior to administration. Do not use if large particulate matter or discoloration is found. If the vaccine is not a homogeneous white suspension, repeat steps 1 and 2. |  |
Step 3. Remove syringe cap Remove the syringe cap from the Luer lock adapter by slowly turning the cap counter‑clockwise while holding the Luer lock adapter.
Note: Care should be taken to ensure that the extended plunger rod is not depressed while removing the syringe cap. |  |
Step 4. Attach a sterile needle Attach a needle appropriate for intramuscular administration to the pre-filled syringe by holding the Luer lock adapter and turning the needle clockwise. | |
Administration
For intramuscular use only.
Each 0.5 mL dose is to be injected intramuscularly, with care to avoid injection into or near nerves and blood vessels. The preferred sites for injection are the anterolateral aspect of the thigh in infants and the deltoid muscle of the upper arm in children and adults. The vaccine should not be injected in the gluteal area.
Do not administer PREVNAR 20 intravascularly.
Resources
Didn’t find what you were looking for?
Contact us
*Contact Medical Information. 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Contact Pfizer Safety to report an adverse event, side effect or concern about the quality of a Pfizer product:
You may also contact the Canada Vigilance Program directly to report adverse events or product quality concerns at 1-866-234-2345 or www.healthcanada.gc.ca/medeffect.