NURTEC ODT 6 Dosage Forms, Strengths, Composition And Packaging
rimegepant
|
Route of Administration |
Dosage Form / Strength/Composition |
Non-medicinal Ingredients |
|---|---|---|
|
Oral |
Orally disintegrating tablet / 75 mg Rimegepant (as rimegepant sulfate) |
Benzyl alcohol, eucalyptol, gelatin, limonene, maize maltodextrin, mannitol, menthol, menthone, menthyl acetate, sucralose, and vanillin. |
NURTEC ODT tablets are white to off-white, circular, debossed with the symbol 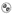 .
.
NURTEC ODT is supplied in a carton, in the following 3 pack sizes:
- blister pack of 2 orally disintegrating tablets (ODTs), in a carton
- blister pack of 8 ODTs, in a carton
- 2 blister packs of 8 ODTs (16 ODTs in total), in a carton.
Find NURTEC ODT medical information:
Find NURTEC ODT medical information:
NURTEC ODT Quick Finder
Health Professional Information
6 Dosage Forms, Strengths, Composition And Packaging
|
Route of Administration |
Dosage Form / Strength/Composition |
Non-medicinal Ingredients |
|---|---|---|
|
Oral |
Orally disintegrating tablet / 75 mg Rimegepant (as rimegepant sulfate) |
Benzyl alcohol, eucalyptol, gelatin, limonene, maize maltodextrin, mannitol, menthol, menthone, menthyl acetate, sucralose, and vanillin. |
NURTEC ODT tablets are white to off-white, circular, debossed with the symbol 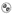 .
.
NURTEC ODT is supplied in a carton, in the following 3 pack sizes:
- blister pack of 2 orally disintegrating tablets (ODTs), in a carton
- blister pack of 8 ODTs, in a carton
- 2 blister packs of 8 ODTs (16 ODTs in total), in a carton.
Resources
Didn’t find what you were looking for?
Contact us
*Contact Medical Information. 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Contact Pfizer Safety to report an adverse event, side effect or concern about the quality of a Pfizer product:
You may also contact the Canada Vigilance Program directly to report adverse events or product quality concerns at 1-866-234-2345 or www.healthcanada.gc.ca/medeffect.