NURTEC ODT 1 Indications
rimegepant
NURTEC ODT (rimegepant orally disintegrating tablets) is indicated for:
- Acute treatment of migraine with or without aura in adults
1.1 Pediatrics
Pediatrics (< 18 years of age): No data are available to Health Canada; therefore, Health Canada has not authorized an indication for pediatric use.
1.2 Geriatrics
Geriatrics (≥ 65 years of age): Clinical trials with rimegepant did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. In pharmacokinetic studies, no clinically significant pharmacokinetic differences were observed between elderly and younger subjects.
)Find NURTEC ODT medical information:
Find NURTEC ODT medical information:
NURTEC ODT Quick Finder
1 Indications
NURTEC ODT (rimegepant orally disintegrating tablets) is indicated for:
- Acute treatment of migraine with or without aura in adults
1.1 Pediatrics
Pediatrics (< 18 years of age): No data are available to Health Canada; therefore, Health Canada has not authorized an indication for pediatric use.
1.2 Geriatrics
Geriatrics (≥ 65 years of age): Clinical trials with rimegepant did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. In pharmacokinetic studies, no clinically significant pharmacokinetic differences were observed between elderly and younger subjects.
2 Contraindications
NURTEC ODT is contraindicated in patients who are hypersensitive to this drug or to any ingredient in the formulation, including any non-medicinal ingredient, or component of the container. For a complete listing, see 6 DOSAGE FORMS, STRENGTHS, COMPOSITION AND PACKAGING.
3 Serious Warnings And Precautions
Serious Warnings and Precautions
Hypersensitivity reactions, including dyspnea and rash, have occurred in less than 1% of patients treated with NURTEC ODT in clinical studies. Hypersensitivity reactions, including serious hypersensitivity, can occur days after administration. If a hypersensitivity reaction occurs, discontinue NURTEC ODT immediately and initiate appropriate therapy (see 2 CONTRAINDICATIONS).
4 Dosage And Administration
4.1 Dosing Considerations
- Hepatic impairment:
The use of NURTEC ODT in patients with severe hepatic impairment should be avoided (see 10.3 Pharmacokinetics).
- Renal impairment:
Use of NURTEC ODT in patients with end-stage renal disease (CLcr < 15 mL/min) and those undergoing dialysis should be avoided (see 10.3 Pharmacokinetics).
4.2 Recommended Dose and Dosage Adjustment
The recommended dose is 75 mg rimegepant, as needed, once daily.
The maximum dose per day is 75 mg rimegepant. The safety of taking more than 15 doses in a 30-day period has not been established.
Geriatric population:
There is limited experience with rimegepant in patients aged 65 years or older. Clinical trials with rimegepant did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients (n=131 treated with rimegepant). In pharmacokinetic studies, no clinically significant pharmacokinetic differences were observed between elderly and younger subjects. (see 7.1.4 Geriatrics, 10.3 Pharmacokinetics).
Hepatic impairment:
No dosage adjustment is required in patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment. Plasma concentrations of rimegepant were significantly higher in subjects with severe (Child-Pugh C) hepatic impairment. Avoid use of NURTEC ODT in patients with severe hepatic impairment (see 7.1.5 Hepatic Impairment, 10.3 Pharmacokinetics).
Renal impairment:
No dosage adjustment is required in patients with mild, moderate, or severe renal impairment. NURTEC ODT has not been studied, and therefore should be avoided, in patients with end-stage renal disease (CLcr < 15 mL/min) and in patients on dialysis (see 7.1.6 Renal Impairment, 10.3 Pharmacokinetics).
4.4 Administration
Instruct the patient on the following administration instructions:
- Use dry hands when opening the blister pack.
- Peel back the foil covering of one blister and gently remove the orally disintegrating tablet (ODT). Do not push the ODT through the foil.
- As soon as the blister is opened, remove the ODT and place on the tongue; alternatively, the ODT may be placed under the tongue.
- The ODT will disintegrate in saliva so that it can be swallowed without additional liquid. NURTEC ODT may be taken with or without food.
- Take the ODT immediately after opening the blister pack. Do not store the ODT outside the blister pack for future use.
5 Overdosage
There is limited clinical experience with NURTEC ODT overdosage. No specific antidote for the treatment of rimegepant overdose is available.
No overdose symptoms have been reported.
Treatment of an overdose of NURTEC ODT should consist of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient. Rimegepant is unlikely to be significantly removed by dialysis because of high serum protein binding.
For management of a suspected drug overdose, contact your regional poison control centre.
6 Dosage Forms, Strengths, Composition And Packaging
|
Route of Administration |
Dosage Form / Strength/Composition |
Non-medicinal Ingredients |
|---|---|---|
|
Oral |
Orally disintegrating tablet / 75 mg Rimegepant (as rimegepant sulfate) |
Benzyl alcohol, eucalyptol, gelatin, limonene, maize maltodextrin, mannitol, menthol, menthone, menthyl acetate, sucralose, and vanillin. |
NURTEC ODT tablets are white to off-white, circular, debossed with the symbol 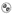 .
.
NURTEC ODT is supplied in a carton, in the following 3 pack sizes:
- blister pack of 2 orally disintegrating tablets (ODTs), in a carton
- blister pack of 8 ODTs, in a carton
- 2 blister packs of 8 ODTs (16 ODTs in total), in a carton.
7 Warnings And Precautions
Please see 3 SERIOUS WARNINGS AND PRECAUTIONS.
7.1 Special Populations
7.1.1 Pregnant Women
There are no adequate human data on the developmental risk associated with the use of rimegepant in pregnancy. Animal studies demonstrate that at clinically relevant exposures rimegepant does not result in embryo-fetal death or fetal malformations. There were no developmental effects in rats at doses up to 60 mg/kg/day (exposures 46 times the human AUC at the maximum recommended human dose [MRHD] of 75 mg/day) or in rabbits at up to the highest dose tested of 50 mg/kg/day (exposures 10 times the MRHD of 75 mg/day). Please see 16 NON-CLINICAL TOXICOLOGY. NURTEC ODT should not be used in pregnant women unless the expected benefit to the mother outweighs the potential risk to the fetus.
7.1.2 Breast-feeding
A lactation study was conducted in 12 healthy adult lactating women (26-37 years of age) who were between 2 weeks and 6 months post-partum and were administered a single oral dose of rimegepant 75 mg. The results have established an average milk-to-plasma ratio of 0.20 and a relative infant dose of less than 1% of the maternal weight-adjusted dose. There are no data on the effects of rimegepant on a breastfed infant or on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for rimegepant and any potential adverse effects on the breastfed infant from rimegepant or from the underlying maternal condition.
7.1.3 Pediatrics
Pediatrics (< 18 years of age): No data are available to Health Canada; therefore, Health Canada has not authorized an indication for pediatric use.
7.1.4 Geriatrics
Geriatrics (≥ 65 years of age): Clinical trials with rimegepant did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients (131 treated with rimegepant). In pharmacokinetic studies, no clinically significant pharmacokinetic differences were observed between elderly and younger subjects (see 4.2 Recommended Dose and Dosage Adjustment).
7.1.5 Hepatic Impairment
Plasma concentrations of rimegepant were significantly higher in subjects with severe (Child-Pugh C) hepatic impairment (see 4.2 Recommended Dose and Dosage Adjustment, 10.3 Pharmacokinetics). The use of NURTEC ODT in patients with severe hepatic impairment should be avoided.
7.1.6 Renal Impairment
Rimegepant has not been studied in patients with end-stage renal disease (CLcr < 15 mL/min) and in patients on dialysis and should be avoided in these patients (see 4.2 Recommended Dose and Dosage Adjustment, 10.3 Pharmacokinetics).
8 Adverse Reactions
8.1 Adverse Reaction Overview
A total of 1771 patients with migraine have been treated with NURTEC ODT or a bioequivalent oral dosage form in the acute treatment of migraine placebo-controlled registration studies. In the overall acute treatment development program, more than 954 patients were exposed for at least 12 months. Most of the adverse reactions reported were mild or moderate in severity.
8.2 Clinical Trial Adverse Reactions
Clinical trials are conducted under very specific conditions. The adverse reaction rates observed in the clinical trials, therefore, may not reflect the rates observed in practice and should not be compared to the rates in the clinical trials of another drug. Adverse reaction information from clinical trials may be useful in identifying and approximating rates of adverse drug reactions in real-world use.
Acute Treatment of Migraine
The safety of NURTEC ODT for the acute treatment of migraine in adults has been evaluated in 3 randomized, double-blind, placebo-controlled trials in 1771 patients with migraine who received one 75 mg dose of NURTEC ODT or a bioequivalent tablet formulation (see 14.1 Trial Design and Study Demographics per Indication, Acute Treatment of Migraine). The most common adverse reaction was nausea. Hypersensitivity, including dyspnea and severe rash, occurred in less than 1% of patients treated with NURTEC ODT.
Adverse reactions are listed by MedDRA system organ class in Table 2.
|
|
NURTEC ODT n = [#] (%) |
Placebo ODT n = [#] (%) |
Rimegepant Tablet* n = [#] (%) |
Placebo Tablet* n = [#] (%) |
|---|---|---|---|---|
|
Gastrointestinal disorders: nausea |
11/682 (1.6%) |
3/693 (0.4%) |
15/1089 (1.4%) |
11/1089 (1.0%) |
* Data for tablet formulation pooled from Studies 2 and 3.
Long-term safety
Long-term safety of rimegepant was assessed in a one-year, open-label study, during which 1197 patients received an average of 6.5 rimegepant tablets per 4 weeks for at least 6 months and 954 received an average of 6.6 rimegepant tablets per 4 weeks for at least 12 months. Approximately 2.6% of subjects experienced a serious adverse event and 2.7% of patients were withdrawn from NURTEC ODT because of an adverse reaction. The most common adverse reaction resulting in discontinuation in the long-term safety study was dizziness. The overall safety profile in the open-label, long-term safety study was consistent with that of the placebo-controlled studies (Study 1, 2, and 3).
8.3 Less Common Clinical Trial Adverse Reactions
Hypersensitivity reactions
Hypersensitivity, including dyspnea and severe rash, occurred in less than 1% of patients treated in clinical studies. Hypersensitivity reactions can occur days after administration, and delayed serious hypersensitivity has occurred.
8.4 Abnormal Laboratory Findings: Hematologic, Clinical Chemistry and Other Quantitative Data
No adverse signals of concern were observed on any of the clinical laboratory assessments in the clinical program for NURTEC ODT.
8.5 Post-Market Adverse Reactions
No new adverse reactions have been identified based on cumulative international post-marketing experience and ongoing Phase 4 clinical trials.
9 Drug Interactions
9.2 Drug Interactions Overview
In Vitro studies
- Enzymes
Rimegepant is a substrate of CYP3A4 and CYP2C9 (see 9.4 Drug-Drug Interactions). Rimegepant is not an inhibitor of CYP1A2, 2B6, 2C9, 2C19, 2D6, or UGT1A1 at clinically relevant concentrations. However, rimegepant is a weak inhibitor of CYP3A4 with time-dependent inhibition. Rimegepant is not an inducer of CYP1A2, CYP2B6, or CYP3A4 at clinically relevant concentrations.
- Transporters
In vitro, rimegepant is a substrate of BCRP and P-gp efflux transporters. Inhibitors of P-gp and BCRP efflux transporters increase plasma concentrations of rimegepant. Rimegepant may be co-administered with BCRP transporter inhibitors and with weak to moderate P-gp only inhibitors. Strong P-gp inhibitors may be co-administered no more frequently than once every 48 hours, based on a clinical interaction study with a potent dual P-gp and BCRP inhibitor (cyclosporine) and with a selective P-gp inhibitor (quinidine) resulted in significant increases of similar magnitude in rimegepant exposure (AUC and Cmax by 1.6 and 1.4 fold with cyclosporine, and by 1.6 and 1.7 fold with quinidine, respectively) (see 9.4 Drug-Drug Interactions).
Rimegepant is not a substrate of OATP1B1 or OATP1B3. Considering its low renal clearance, rimegepant was not evaluated as a substrate of the OAT1, OAT3, OCT2, MATE1, or MATE2-K.
Rimegepant is not an inhibitor of P-gp, BCRP, OAT1, or MATE2-K at clinically relevant concentrations. It is a weak inhibitor of OATP1B1 and OAT3.
Rimegepant is an inhibitor of OATP1B3, OCT2, and MATE1. No clinical drug interactions are expected for NURTEC ODT with these transporters at clinically relevant concentrations (see 9.4 Drug-Drug Interactions).
9.3 Drug-Behavioural Interactions
The effect of rimegepant on sexual activity, driving, and operating machinery has not been studied.
The interaction of rimegepant with cigarette smoking, cannabis use, and/or alcohol consumption has not been studied.
9.4 Drug-Drug Interactions
| [Proper/Common name] | Source of Evidence | Effect | Clinical Comment |
|---|---|---|---|
| CYP3A4 Inhibitors (e.g., clarithromycin, itraconazole, ritonavir, diltiazem, erythromycin, fluconazole, grapefruit juice) | In vivo | Concomitant administration of 75 mg rimegepant (single dose) with itraconazole, a strong CYP3A4 inhibitor, at steady state resulted in increased exposures of rimegepant (AUC by 4-fold and Cmax by ~1.5-fold). The concomitant administration of rimegepant with a moderate inhibitor of CYP3A4 (e.g., fluconazole), at steady state, increased rimegepant exposures (AUC) by 1.8-fold. No dedicated drug interaction study was conducted to assess the effect of concomitant administration of a weak inhibitor of CYP3A4 on the pharmacokinetics of rimegepant. Concomitant administration of rimegepant with a weak inhibitor of CYP3A4 is not anticipated to have a clinically significant impact on rimegepant exposures. | Concomitant administration of NURTEC ODT with strong CYP3A4 inhibitors (e.g., clarithromycin, itraconazole, ritonavir) should be avoided . Exercise caution during concomitant administration of rimegepant with moderate CYP3A4 inhibitors (e.g., diltiazem, erythromycin, fluconazole). Another dose of NURTEC ODT within 48 hours should be avoided when it is concomitantly administered with moderate inhibitors of CYP3A4 (e.g., fluconazole). |
| CYP3A4 Inducers (e.g., phenobarbital, rifampicin, St John’s wort (Hypericum perforatum), bosentan, efavirenz, modafinil) | In vivo | Concomitant administration of 75 mg rimegepant (single dose) with rifampin, a strong CYP3A4 inducer, at steady state, resulted in decreased exposures of rimegepant (AUC by 80% and Cmax by 64%), which can lead to loss of efficacy. The effects of moderate or weak inducers of CYP3A4 on the pharmacokinetics of rimegepant are unknown. However, since rimegepant is a substrate for CYP3A4, drugs that are moderate inducers of CYP3A4 can also cause significant reduction in rimegepant exposure resulting in loss of efficacy. Clinically significant interaction is not anticipated with concomitant administration of weak inducers of CYP3A4 and rimegepant. | Concomitant administration of NURTEC ODT with strong CYP3A4 inducers (e.g., phenobarbital, rifampicin, St John’s wort (Hypericum perforatum)) or moderate CYP3A4 inducers (e.g., bosentan, efavirenz, modafinil) is not recommended. The effect of CYP3A4 induction may last for up to 2 weeks after discontinuation of the strong or moderate CYP3A4 inducer. |
| BCRP and/or P-gp only Inhibitors (e.g., cyclosporine, verapamil, quinidine). | In vivo | Concomitant administration of 75 mg rimegepant (single dose) with a single 200 mg dose of cyclosporine, a strong inhibitor of the P-gp and BCRP transporters, resulted in 1.6-fold and 1.4-fold increase in rimegepant AUC and Cmax, respectively. Upon concomitant administration of a single 75 mg dose of rimegepant and a single 600 mg dose of quinidine, a strong inhibitor of P-gp only, rimegepant AUC and Cmax increased by approximately 1.6 fold and 1.7 fold, respectively. Based on the totality of the results, BCRP inhibition is not anticipated to significantly affect rimegepant exposures. | Avoid a second dose of NURTEC ODT within 48 hours after concomitant administration of the first dose with strong inhibitors of P-gp (e.g., cyclosporine, verapamil, quinidine). |
Other drugs
CYP2C9 inhibitors:
Rimegepant is primarily metabolized by CYP3A4 and to a lesser extent by CYP2C9. Increase in the exposure of rimegepant can be attributed to combined inhibition of CYP2C9 and CYP3A4. In a drug-drug interaction study in healthy adult subjects, administration of a single 75 mg dose of rimegepant concurrent with steady state fluconazole (400 mg QD) increased AUC of rimegepant by approximately 1.8-fold while its Cmax did not change significantly.
MATE1 substrates:
In a dedicated drug interaction study in healthy nondiabetic adult subjects, steady state administration of rimegepant 75 mg with metformin 500 mg, a MATE1 transporter substrate, at steady state, resulted in no clinically significant impact on either metformin pharmacokinetics or on glucose utilization.
CYP3A4 substrates and pharmacodynamic interactions:
In dedicated drug interaction studies in healthy adult subjects, rimegepant did not have a clinically significant effect on the pharmacokinetics of oral contraceptives (norelgestromin, ethinyl estradiol or midazolam (a sensitive CYP3A4 substrate) (see 10.2 Pharmacodynamics).
In another drug interaction study in healthy adult subjects, steady state administration of oral rimegepant 75 mg did not affect sumatriptan (OCT1 substrate) pharmacokinetics (2 x 6 mg administered subcutaneously within one hour). Also, single-dose administration of sumatriptan did not have any meaningful effect on rimegepant pharmacokinetics.
Other calcitonin gene-related peptide (CGRP) Receptor Antagonists:
In a clinical study of two other CGRP receptor antagonists, a significant increase in cases of constipation was reported when these CGRP receptor antagonists were co-administered. Concomitant use of rimegepant with other CGRP receptor antagonists is not recommended.
9.5 Drug-Food Interactions
Following administration of NURTEC ODT under fed conditions Tmax was delayed by approximately 1 to 1.5 hours. Consumption of a high-fat meal 30 minutes before administration of NURTEC ODT under or on top of the tongue decreased rimegepant AUCT by 32% and 38%, and Cmax by 42% and 53%, respectively. Similarly, consumption of a low-fat meal 30 min before administration of NURTEC ODT under the tongue decreased rimegepant AUCT and Cmax by approximately 28% and 36%, respectively, compared with administration under fasting conditions.
NURTEC ODT was administered without regard to food in clinical safety and efficacy studies. It is not known whether there is any impact on efficacy when there is a reduction in rimegepant exposure when administered with food.
9.6 Drug-Herb Interactions
See 9.4 Drug-Drug Interactions regarding concomitant use with St. John’s wort (a CYP3A4 inducer).
9.7 Drug-Laboratory Test Interactions
Interactions of NURTEC ODT with laboratory tests have not been studied.
10 Clinical Pharmacology
10.1 Mechanism of Action
Rimegepant is a calcitonin gene-related peptide receptor antagonist. Rimegepant selectively binds with high affinity to the human calcitonin gene-related peptide (CGRP) receptor and antagonizes CGRP receptor function.
The relationship between pharmacodynamic activity and the mechanism(s) by which rimegepant exerts its clinical effects is unknown.
10.2 Pharmacodynamics
No clinically relevant differences in resting blood pressure were observed when rimegepant was concomitantly administered with sumatriptan (12 mg subcutaneous, given as two 6 mg doses separated by one hour) compared with sumatriptan alone to healthy volunteers.
Abuse liability has not been studied in humans or in animals.
Cardiac Electrophysiology
In a double-blind, randomized, placebo- and positive-controlled, crossover ECG assessment study in healthy subjects (N=38), single 75 mg and 300 mg doses of rimegepant did not show any pharmacodynamic effect on the QTc interval.
10.3 Pharmacokinetics
CYP2C9 polymorphism
Rimegepant Cmax and AUC0-inf were similar in CYP2C9 intermediate metabolizers (i.e., *1/*2, *2/*2, *1/*3, n=43) as compared to normal metabolizers (i.e., *1/*1, n=72). Adequate PK data are not available from CYP2C9 poor metabolizers (i.e., *2/*3, n=2). Since the contribution of CYP2C9 to rimegepant metabolism is considered minor, CYP2C9 polymorphism is not expected to significantly affect its exposure.
Absorption
Following oral administration of NURTEC ODT, rimegepant is absorbed with the maximum concentration at 1.5 to 2.0 hours. The absolute oral bioavailability of rimegepant is approximately 64%.
Distribution:
The steady state volume of distribution of rimegepant is 120 L. Plasma protein binding of rimegepant is approximately 96%.
Metabolism:
Rimegepant is primarily metabolized by CYP3A4 and to a lesser extent by CYP2C9. Rimegepant is the primary form (~77% ) with no major metabolites (i.e., > 10%) detected in plasma.
Elimination:
The elimination half-life of rimegepant is approximately 11 hours in healthy subjects. Following oral administration of [14C]-rimegepant to healthy male subjects, 78% of the total radioactivity was recovered in feces and 24% in urine. Unchanged rimegepant is the major single component in excreted feces (42%) and urine (51%).
Special Populations and Conditions
- Geriatrics: In pharmacokinetic studies, no clinically significant pharmacokinetic differences were observed between elderly (≥ 65 years) and younger (18-45 years) subjects.
- Sex: No clinically significant differences in the pharmacokinetics of rimegepant were observed based on sex.
- Genetic Polymorphism: No clinically significant differences in the pharmacokinetics of rimegepant were observed based on CYP2C9 genotype.
- Ethnic Origin: No clinically significant differences were observed in studies where the pharmacokinetics of rimegepant in White, Black, and Japanese study participants were assessed.
- Hepatic Insufficiency: In a dedicated clinical study comparing the pharmacokinetics of rimegepant in subjects with mild, moderate, and severe hepatic impairment to that with normal subjects (healthy matched control), the exposure of rimegepant (Cmax and AUC) following single 75 mg dose was approximately 2-fold higher in subjects with severe impairment (Child-Pugh class C). There were no clinically meaningful differences in the exposure of rimegepant in subjects with mild (Child-Pugh class A) and moderate hepatic impairment (Child-Pugh class B) compared to subjects with normal hepatic function (see 4.2 Recommended Dose and Dosage Adjustment, Hepatic impairment).
- Renal Insufficiency: In a dedicated clinical study comparing the pharmacokinetics of rimegepant in subjects with mild (estimated creatinine clearance [CLcr] 60-89 mL/min), moderate (CLcr 30-59 mL/min), and severe (CLcr 15-29 mL/min) renal impairment to that with normal subjects (healthy matched control), total rimegepant exposure was increased by 6%, 40%, and 4%, respectively following a single 75 mg dose. NURTEC ODT has not been studied in patients with end-stage renal disease (CLcr < 15 mL/min) and in patients undergoing dialysis (see 4.2 Recommended Dose and Dosage Adjustment, Renal impairment).
- Obesity: No clinically significant differences in the pharmacokinetics of rimegepant were observed based on body weight.
11 Storage, Stability And Disposal
Store NURTEC ODT at room temperature (15°C to 30°C).
12 Special Handling Instructions
Instructions for handling the medicinal product before administration are:
- Use dry hands when opening the blister pack.
- Peel back the foil covering of one blister and gently remove the orally disintegrating tablet. Do not push the tablet through the foil.
- Take the tablet immediately after opening the blister pack. Do not store the tablet outside the blister pack for future use.
Control #: 268384
DEC 1, 2023
Resources
Didn’t find what you were looking for?
Contact us
*Contact Medical Information. 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Contact Pfizer Safety to report an adverse event, side effect or concern about the quality of a Pfizer product:
You may also contact the Canada Vigilance Program directly to report adverse events or product quality concerns at 1-866-234-2345 or www.healthcanada.gc.ca/medeffect.